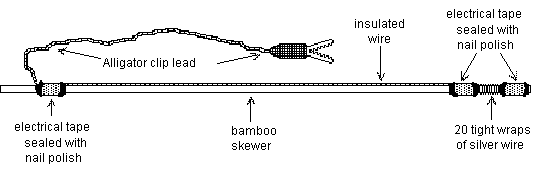BIO325 Laboratory Guide #3 (2024)
ELECTROPHYSIOLOGY I:
RECORDING FROM WEAKLY ELECTRIC FISH
falls under category
A
Electrosensory or "weakly electric" fish typically have very poor eyesight and live in turbid water. Their primary sensory modality involves producing a weak electric field around their bodies, then detecting the distortions in this field caused by nearby conductive or nonconductive objects. Electroreception of this type involves an organ for producing the electrical discharge and special sensory organs for detecting it. The electrical organ is essentially a set of giant, highly-modified neuromuscular junctions, which generate giant, highly-modified postsynaptic potentials into the water. These signals are detected by pit-shaped skin receptors distributed over the body, and processed by a modified region of the lateral line lobe (inferior colliculus).
Weakly electric fish come in two basic flavors - "wave-type" and "pulse-type". There are several genera and species of each type of fish. Wave-type fish produce a continuous, nearly sinusoidal signal at a characteristic frequency. Pulse-type fish produce discrete pulses into the water, somewhat like the ultrasonic cries of bats and the sonar pulses of dolphins. You will be working first with a wave-type fish called a green knifefish Eigenmannia viriscens, then with a pulse-type fish called an elephant nose Gnathonemus. Recording the relatively simple, "user-friendly", electrical signals produced by these fish provides an interesting way to learn about some practical considerations of electrophysiological recording.
But first, a short digression on electrodes. Assuming that you have two wire leads coming off of a recording or stimulating circuit, what is an appropriate way to make contact with your fish? On first thought, it might seem sufficient to just immerse the free end of each wire in the aquarium with your fish. If bare wires were used without electronic filtering, a slowly drifting potential difference would be observed between the wires. This potential would be erratic, bear little relationship to the actual electrical activity of the fish, and seriously compromise your recording. When a bare metal electrode is immersed in a saline solution, an electrical potential is established between the metal and the solution, due to the metal yielding ions to the solution, and/or the solution plating ions onto the metal. These are called electrode or junction potentials and are one of the banes of electrophysiology (the other being electromagnetic noise). If the junction potentials at the two electrodes were constant and identical, they would cancel out in the overall potential measurement. In order for this to happen, both the conditions at the surface of each electrode, and the ionic makeup of the solution surrounding each electrode would have to be constant and identical. However, as current flows in a single direction through each junction, the properties of the junction change, e.g. as a result of electroplating of ions onto the electrode. This process is compounded when electrodes are used to pass externally applied, steady "DC" currents. Under such conditions, bare metal electrodes can rapidly "polarize" and block further current flow.
For future labs, involving the recording of slowly changing or steady "DC" potentials, we will have to use metallic silver electrodes which have been electroplated with a layer of silver chloride and surrounded by a saturated potassium chloride solution. When placed into the predominantly NaCl salt solution of tissue, the Ag - AgCl – KCl – NaCl interface remains fairly stable and can accurately record and pass direct currents.
However, in this lab you will be recording a rapidly changing "AC" signal. Bare metal electrodes are, in fact, adequate because you will be electronically filtering out the low-frequency components of the signal, including the baseline drift described above. You will construct recording/stimulating electrode wands with an exposed silver coil at the business end. Silver is used because it is fairly non-corrosive and biologically inert. The most serious considerations are 1) that the larger the exposed metal surface of the recording lead, 2) the closer this recording surface is to the fish, and 3) the more completely shielded your fish and leads are from radio-frequency (RF) noise, the better the recording. To shield out RF noise the aquarium, fish, and recording electrode wands will all be inside a grounded wire-screen Faraday cage. The electrode wands themselves will also be wrapped in a grounded shield of conductive aluminum foil.
I. CONSTRUCTING SIMPLE WAND ELECTRODES
Follow the instructions and the diagram at the right to construct two wand electrodes.
1) Break the sharp end off of a bamboo skewer. Smooth the broken end with sandpaper or a file.
2) Carefully and tightly wrap a silver wire ~20 times around the skewer 1/2 cm in from one end. Make sure that your wraps lie next to each other and don't overlap - this will create a large, stable recording surface and reduce recording noise. Cut both free ends of the silver wire off at about 1 cm length.
3) Cut an alligator clip lead in half and deinsulate the cut ends.
4) Cut a length of insulated hookup wire about 5 cm shorter than your skewer. Deinsulate one end and splice it to the inner free end of the silver wire by tightly twisting the wires together. Deinsulate the other end of the hookup wire and splice it to the one end of the alligator clip lead.
5) Fold over the outer end of the silver wire and secure it to the skewer using electrical tape. Stretch the hookup wire the length of the skewer and secure both of its spliced ends to the skewer with electrical tape. Make sure that your tape wrappings completely cover the splice points and are smooth with no folds or wrinkles.
6) Seal both sides of each tape wrapping with nail polish. After 5 minutes, make another nail polish application. It is critical that all of these seals be complete and water-tight.
7) Allow at least 20 minutes for the nail polish to dry.
8) Wrap the entire wand except the exposed silver wire coil in aluminum foil. Make very sure that he foil does not touch the silver recording coil.
II. WAVE-TYPE FISH
A. Initial Recording Setup
1) Start up the PC (if necessary) and set up the screen capture utility. Turn on the PowerLab box, and launch the Scope application.
2) For this lab you will share a four-channel model 1700 AC amplifier with the other recording station at your table. Connect your two electrodes to either Channel 1 or 4 of the amplifier by connecting their alligator clips to the two central wires of the special amplifier cable lead. Note that the shield on this lead is actively driven and should NOT be connected to either the aquarium water or to the amplifier ground. Connect the aluminum foil shields of your wands to the Faraday cage with long alligator clip wires.
3) Connect the output of your amplifier channel to the CH1+ input of the PowerLab, using a BNC cable and BNC T-connector. Connect off of this T-connector through the audio source selector switch (set to STR) to one tuner channel on the back of the audio amplifier using a BNC-RCA phono plug adapter and phono plug cables.
4) Set up the PowerLab for monopolar AC recording on Channel A (CH1+) only. To do this, open the Input Amplifier . . . box for Channel A, set the Range to 5V, and check the AC and Positive boxes. You can eliminate the display for Channel B entirely by pulling down the Display window, choosing Computed Functions . . . , then choosing Channel A Only under Display. Set the Mode for Repetitive sweeps, by choosing Sampling under the Setup menu.
5) Set the AC amplifier channel gain to the lowest setting (x100), the filters to wide open (0.1 Hz low-cutoff and 20 kHz high-cutoff), the notch filter to out, and the mode to Rec.
6) Turn on the AC amplifier.
7) Set the audio amplifier volume to low, mono on, tuner selected. Turn on the power and disconnect the headphones.
8) Remove the tubes and obstacles from the aquarium.
9) Start the PowerLab sweeps, lower your wands into the water, and record from your fish. Try different sweep speeds (using the Time Base window), and different amplifications (using the Input A window) - you are looking for a signal whose dominant frequency is about 300 Hz for Eigenmannia. When you are ready to save some traces, reset the sampling Mode of PowerLab to Multiple and save some traces.
10) When you are sure that you have the necessary traces to complete the following data sheet item, save the file to disk, then procede with C below. DO NOT TAKE THE TIME TO PRINT OUT YOUR RESULTS NOW.
B. Orientation of the Electric Field
For this part of the experiment you will need to make and save several recordings to answer the following questions. To do this you may need to chase the fish around with your recording wands. Each time you collect a good trace, be sure to label it clearly using the internal Scope memo pad feature.
Q1: How is the electric field oriented relative to the body of the fish - i.e. is the recording strongest when the electrodes are on either end of the fish or on either side of the fish? What does this tell you about the probable shape of the EOD field?
Q2: Where is the electric organ itself - i.e. around what part of the fish is the EOD the strongest? Does the fish exhibit any behaviors which support your conclusion?
C. Amplifier Filtering
For this part of the experiment you will need to make and save several recordings with the electrodes in a fairly constant orientation relative to the fish. Adjust the low-frequency cutoff, high-frequency cutoff, and 60 Hz notch filters on the AC amplifier and evaluate the effects on the recorded signal. As you collect the data for each question below, label each saved trace using the Scope memo pad feature. Each label should specify the amplification, filter settings, and electrode locations relative to the fish.
Q3: Does turning on the 60 Hz notch filter significantly alter the recorded signal? Why or why not?
Q4: Set the high-pass (low-frequency cutoff) filter to successively higher frequency settings. How does this effect the signal? Does this improve the background noise and/or the baseline stability?
Q5: Set the low-pass (high-frequency cutoff) filter to successively lower frequency settings. How does this effect the signal? Does this improve the background noise and/or the baseline stability?
Q6: What are the ideal amplifier filter settings for this recording -i.e. those which produce minimal distortion in the recorded signal, while minimizing unwanted high-frequency "shot” noise, 60 Hz AC line interference, and slow "DC" baseline drift?
D. Jamming Avoidance Response (JAR)
When two knifefish come into close proximity in the wild, each fish senses not only its own signal, but that of its neighbor. Since the signals are continuous, and at nearly the same frequency, there is the potential for each fish to inadvertently interfere with or “jam” the electroreception system of its neighbor. Knifefish respond to potentially jamming signals by very rapidly shifting their own signal frequency 1-10 Hz away from the potentially jamming signal. This shift is called the “jamming avoidance response (JAR)”.
Set your amplifier settings to the “ideal” settings which you determined in the previous section. Record a sample for each of two fish in isolation. Now put them together in the same tank. Chase each fish around with the wands to again record from each fish. If you are patient you should be able to get a relatively clean record off of each fish in the paired situation.
Q7: Does either fish shift its frequency noticeably when the fish are paired? Do the changes result in the signal frequencies shifting away from each other?
E. Shutting Down If you have not recorded from the pulse-type fish, go on to the next section. Otherwise, make sure that you have saved all of your data, then quit Scope. Turn off the PowerLab box and the sine wave generator. Disconnect the cables and the electrode wands.
III. PULSE-TYPE FISH
A. Initial Recording Setup
The recording setup and considerations for a pulse-type fish are very similar to what you used for the wave-type fish. You should be familiar enough with the PowerLab system to adapt the setup without detailed instructions. Scope, set for either repetitive or multiple sweeps, will probably work better than Chart.
B. Recording From a Pulse-Type Electric Fish.
Follow Crawdad Lab 1, pages 4-5 for this part of the lab. Refer to the CD-ROM guide for help with the set-up.
C. Shutting Down
If you have not recorded from the wave-type fish, go on to the next section. Otherwise, make sure that you have saved all of your data, then quit Scope or Chart. Turn off the PowerLab box. Disconnect the cables and the electrode wands.
IV. PREPARATION OF THE LAB DATA SHEET
Your data sheet should include all SIX of the items described in the boxes above. Make sure that the axes of all of the graphs and print-outs are labeled and calibrated. You should certainly discuss your results and the answers to the questions with your partners and others in the lab. However, please work independently when you prepare your data sheet.
The writeup
for this lab
falls under category
A