BIO112 Laboratory Guide #4
DIVERSITY OF THE FUNGI
INTRODUCTION.
Once upon a time the members of the Kingdom Fungae (or Fungi) were lumped together in the same kingdom with the plants. Both groups are sessile and have cell walls. In addition, both groups are eukaryotic, lack centrioles in their cells, and are mostly multicellular. The similarities that seem to unite these two groups of organisms disappear on closer inspection, however. Fungi have no motile cells at all in their life cycles (with one exception), whereas in plants the sperm are motile (except in the flowering plants, where this trait has been lost secondarily). Furthermore, although both have cell walls, they are composed of different kinds of polysaccharides: chitin in fungi (same as in arthropod exoskeletons!), cellulose in plants.
The fungi are heterotrophs that absorb their food after digesting it with extracellular enzymes. The structure of a fungus is essentially filamentous, rather than truly three dimensional as in the plants. Fungi are composed of threadlike structures called hyphae, which in aggregate form a mycelium. Some aspects of cellular structure in Fungi are quite unusual: the nuclear envelope does not break down in cell division, and the spindle is formed within the nucleus. Also, the cell walls between cells are often incomplete, allowing nuclei to move from cell to cell. Therefore, fungal cells can be multinucleate. In spite of this simple basic mycellar mat structure, some fungal groups produce complex reproductive structures, and these provide the morphological basis for their classification into five Divisions (Phyla).
Fungi occupy a wide range of ecological roles. Most fungi are saprophytic and adsorb organic nutrients from dead organisms as part of the decay process. For the parasitic and pathogenic fungi the food source is a living plant or animal and the fungus must overcome the biochemical defense mechanisms of the host organism. There are even examples of predatory fungi, for example a fungus that traps nematode worms in hyphal loops and slowly consumes them! Fungae are also involved in number of symbiotic, mutualistic relationships, including the nutritional relationships mycorrhizae have with plant roots and the extremely intimate interactions of the compost organisms known as lichens.
In this lab you will encounter representatives of Kingdom Fungae and will learn about their basic biology, ecology, and systematics. You will also conduct an experiment testing the role that symbiotic mycorrhizae play in plant nutrition and growth.
After completing this laboratory you should be able to:
1) List and define the distinguishing characteristics of members of the five Phyla (Divisions) of fungi;
2) Identify and correctly classify the specimens seen in this laboratory;
3) Identify, describe, and provide examples for the various ecological roles that fungi play;
4) Characterize the specific interactions between plant roots and mycorrhizal fungi as a mutualistic, symbiotic relationship.
PART I. DIVERSITY OF THE FUNGI
Materials
Specimens at numbered stations arranged on the lab benches.
These consist of fresh materials and live specimens as well as preserved specimens, prepared microscope slides, and other written and illustrated materials.
Microscopes, slides, cover slips, forceps, lens paper.
Dissecting stereomicroscopes.
Procedure
1. View the specimens of fungi, noting their key identifying features. A good idea for future studying would be to make a drawing of each specimen, labeling all of the characteristic structural features.
2. For each specimen, be sure to record its common name as well as its formal classification within the fungi - specifically the fungal Division (Phylum) to which it belongs.
3. Be able to recognize all specimens as well as their distinguishing characteristics.
Be able to describe the general ecology of each specimen, e.g. is it a free living saprobe, a parasite, a pathogen, a mutualistic symbiote?
4. For the live specimens, make a wet mount of each, or examine a prepared slide, or look at an entire specimen, depending on the organism.
Study Suggestions
1. Make detailed sketches and notes on specimens. This will help you in two ways: 1) when you attempt to draw a specimen, you are forced to look at it more closely, and 2) the drawings will help you study later.
2. Plan to come view the specimens once or twice more before the lab test. Test yourself by attempting to identify the specimens without first looking at their labels.
A. FUNGAL DIVISIONS
There are five divisions in the Kingdom Fungae: Chytridiomycota, Zygomycota, Glomerulomycota, Ascomycota, and Basidiomycota. (Note that use of the term “Division,” rather than “phylum,” is a throwback to the days when fungi were lumped with plants; plant phyla are also called Divisions.) The naming also can be a bit confusing - "Fungae and -mycotes" generally refer to the formal taxa, while "fungi" and -mycetes" apply to members of those taxa. Fungal Divisions are distinguished by differences in their life cycles and in the structures associated with reproduction. Create a table in your lab manual summarizing the distinguishing features of each of the following five groups. Arrange the table as follows:
DIVISION
Characteristic
Structural
Features
Diagnostic
Reproductive
Features
Ecology
Examples of
Species in
This Group
Chytridiomycota
Zygomycota
Glomerulomycota
Ascomycota
Basidiomycota
Chytridiomycota (chytrids)
The chytrids, such as Chytridium, were formerly lumped with the protists, but molecular and structural evidence places them in kingdom Fungae. They have chitinous cell walls, have an absorptive mode of nutrition, and some species form coenocytic hyphae. These species do, however, have spores with a single flagellum, unlike all other fungi. The chytrid lineage is believed to represent the most primitive group of fungi, and probably evolved from flagellated protists. The chytrids are mostly aquatic and are saprobes and parasites.
View the prepared slide of Allomyces, which is a whole mount of the sporophyte.
Zygomycota (true molds)
In this group of fungi, the hyphae lack cross walls. An example is the black bread mold, Rhizopus nigricans. Zygotes are produced sexually and are the only diploid stage of this fungus. The thick-walled structure around the zygote is the zygosporangium, which prevents desiccation of the zygote. Spores are produced asexually by sporangia borne on stalks.
View the prepared slide of Rhizopus zygosporangia, the round purple-brown structures between green fungal hyphae.
Glomerulomycola (arbuscular fungi)
The glomerulomycetes were formerly lumped with the zygomycetes, based on their spherical sporangia. Almost all members of this group form symbiotic endomycorrhizae relationships with plant root cells. The fungal hyphae extend into the cells and form branching "arbuscles". In this symbiotic relationship the fungi provide basic soil nutrients to the plant, while receiving photosynthetic products back from the plant.
View the prepared slide of endomycorrhizae. In this slide the fungal arbscules are stained reddish purple with the green root cell walls.
Ascomycota (sac fungi)
The defining feature of the sac fungi is that the spores (ascospores) are borne inside a sac-like structure called the ascus (plural: asci). Also, the hyphae have perforated cross walls separating the cytoplasm of adjacent cells. Examples of ascomycetes are yeasts, mildew, Neurospora, morels, and truffles. The fruiting body of ascomycetes, which is often cup-shaped, is called an ascocarp.
View the prepared slide of Morchella with asci. This is the morel, a much-sought after edible mushroom often found beneath oak trees.
The ascomycetes are an extremely diverse group, including the free-living, sexually reproducing sac fungi such as morels, as well a wide range of asexually reproducing forms which lack these characteristic ascosporangia.
The yeasts are single-celled fungi of tremendous economic significance. Yeasts undergo anaerobic respiration, producing carbon dioxide gas and alcohol as waste products. The bubbles in champagne, beer, and rising bread are made of carbon dioxide. Alcohol produced by yeasts in a closed container eventually builds up to toxic levels, killing the yeasts and putting a halt to ethanol production -- this is why wine naturally has an alcohol content of only about 12 percent.
Put a drop of live yeast cells on a slide, then view them under the microscope. You may be able to see yeast cells “budding” off - this is how they asexually reproduce.
Penicillium is another asexually reproducing ascomycete. View the prepared slide of Penicillium and note the strings of conidia asexually budding off of the hyphal strands.
Basidiomycota (club fungi)
The characteristic feature of this group is the basidium (a club-like structure on a stalk), which contains the basidiospores within it. Often the basidia are borne on gills or within pores. Like the sac fungi, hyphae have perforated cross walls separating cells. Examples of basidiomycetes are the mushrooms and rusts.
Examine the specimens of the commercial mushroom (Agaricus bisporus): note the gills underneath the cap. The mushroom is the fruiting body (or basidiocarp) of the fungus; most of the mycelium is actually underground. Remove a segment of gill and mount it on a microscope slide. Can you see the basidia and basidiospores? Also slice a stem lengthwise and try to see the filamentous structure of the mushroom.
Next view a prepared slide of Coprinus. Here the basidia should be plainly visible.
Deuteromycota (fungi imperfecti)
This former polyphyletic group includes species which lack sexual reproduction, making classification based on reproductive structures impossible. Molecular systematics and detailed analysis of cells structure have reclassified most of the deuteromycotes as members of Ascomycota.
B. FUNGAL ECOLOGY
Fungi occupy diverse ecological roles in biological communities. Many fungi are decomposers, i.e., saprobes, subsisting on dead organic matter. Fungi are among the few organisms that can break down cellulose or lignin, the major components of fallen tree trunks. View the prepared slide of the woodrot fungus, which shows the hyphae penetrating the wood. The hyphae have lots of surface area for absorption of nutrients, and so are ideal structures for decomposers.
Many agricultural diseases are caused by parasitic or pathogenic fungi. For example, the bracket fungus on display can penetrate the living tissues of trees. The rusts are a very damaging group of parasites. View the slide of the basidiomycete wheat rust Puccinia graminis showing the telia stage (one of a number of different stages of its very complex life cycle). Can you see the fungal spores? Also see the preserved wheat stems showing wheat rust stages. There is also a preserved specimen of the ascomycete Claviceps purpurea (ergot) on wheat. The economic costs of fungi destroying human foods are staggering.
Not all fungi have sinister lifestyles, however. Many fungi engage in symbiotic mutualisms with other organisms. For example, lichens are mutualisms between a cyanobacterium or green alga and a fungus, usually an ascomycete. Lichens can tolerate incredibly harsh conditions, and are often the dominant organism in extreme habitats such as Antarctica, where they grow on rocks.
Examine the specimens of lichen.
PART II: MYCORRHIZAL SYMBIOSIS
The ecological relationship between plants and the fungi that colonize their roots is both ancient and widespread. Fossils of the earliest land plants, dated at over 400 million years old, have evidence of fungal hyphae associated with their roots. About 90% of plant species have mycorrhizae, the name for the fungal structures that grow on and in plant roots.
But what do these fungi do, exactly? Mycorrhizae are an example of a symbiosis, an ecological relationship between two species in which they are intimately associated with each other. Some but not all symbioses are also mutualistic, meaning that both species involved in the interaction benefit from the presence of the other. Mycorrhizae are beneficial to plants because they help roots to acquire mineral elements from the soil (e.g., phosphorus); in turn, the fungus gets carbohydrates produced by the plant during photosynthesis. (Can you think of an actual example where two species exhibit a non-mutualistic symbiosis?)
The vast majority of mycorrhizae are from the phylum Glomeromycota in the Kingdom Fungi. These fungi form arbuscular mycorrhizae (formerly called “endomycorrhizae”), where the hyphae make microscopic branching structures (arbuscules) inside (thus, “endo-“) root cells. Another kind of mycorrhizae, ectomycorrhizae, is characteristic of the phyla Zygomycota, Basidiomycota, and Ascomycota. In ectomycorrhizae, the fungal hyphae form a sheath surrounding the plant root and may grow between cortical cells of the root, but do not actually penetrate cell walls. The table below summarizes the kinds of mycorrhizae and the types of plants with which they form symbiotic relationships.
Kingdom Fungi and Mycorrhizae
Fungal Phylum
Type of Mycorrhizae*
Plants Associated with
these Mycorrhizae
Chytridiomycota
None
N/A
Zygomycota
Ectomycorrhizae
Mostly trees
Basidiomycota
Ectomycorrhizae
Mostly trees
Ascomycota
Ectomycorrhizae
Mostly trees
Glomeromycota
Arbuscular Mycorrhizae
Grasses, other non-woody plants, some trees
In this part of the lab, you will carry out a multi-week experiment testing the effects of fungal mycorrhizae on plant growth, then you will stain and view fungi microscopically.
A. SOIL PREPARATION AND PLANTING
Materials
plastic pots: 8 per group or 4 students
pea gravel
playground sandorganic soil (no additives), baked at 204oC for 1 hour to kill soil microbes and fungi
organic seeds of one species form each of the following two plant groups:
mycorrhizal-dependent species: marigold, bean, cucumbernon-mycorrhizal-dependent species: broccoli, mustard, radish
commercial mycorrhizal inoculum:
Arbico Organics Root Maximizer Mycorrhizal Fungi (Arbico-Organics.com)hand trowels
watering cans
¼ teaspoon measure
sharpies
label tape
Procedure
The methods and general design of this exercise are modified from Johnson et al. (2009). The question you will address in this exercise is “how does the presence of mycorrhizae influence growth of plants?” To address this question, you grow plants for several weeks under identical rearing conditions, using soil under two treatment conditions:
treatment 1 - experimental - with commercial mycorrhizae added
treatment 2 - control - without commercial mycorrhizae .
As a group of 4 students you will will be assigned two plant species:
species 1 - a mycorrhizal-dependent species (marigold, bean, cucumber)
species 2 - a non-mycorrizal-dependent species (broccoli, mustard, or radish)
Your group will use 2 replicate pots per treatment/species combination - for a total of eight pots per lab group.
Start by obtaining 8 pots and labeling each one with your group initials, the plant species, and the soil treatment (experimental or control). The instructor will demonstrate the specific method for preparing each pot and sowing the seeds. You will sow 5 seeds per pot.
When you have finished sowing all of the seeds in all eight pots, gently water each one until water flows out of the bottom of the pot. When all of your pots have been watered, move them to your designated space in the Green Knights Greenhouse on the third floor (roof) of the Munroe Science Center.
B. PLANT MAINTENANCE
For the next several weeks, you will need to check your plants daily and water them as necessary to keep the soil moist.
At the end of 3 weeks you will need to thin each pot by carefully removing all but one of the growing plants. Try to keep the largest/most centrally located plant in each pot.
After 7 weeks you will harvest and measure your plants during the lab period for Lab #9: Diversity of the Plants, as described below.
C. HARVESTING AND MEASURING PLANTS
Materials
newspaper or aluminum trays
rulers (cm) or electronic calipers
analytical balance
small scissors
forceps
Procedure
During Lab #9 you will harvest your plants and measure two dependent variables for each: plant height (cm) and total plant mass (g; including all leaves, stems, roots).
Keeping careful track of which treatment each plant came from, do the following for each plant:
1. First use the ruler or calipers to measure the total height (to the nearest mm)
from the soil level to the tallest point of the plant. Record this datum to your
data sheet.
2. Over a tray or newspaper carefully tip out the contents of the pot. Gently
shake off the dirt.
3. Rinse the roots in tap water, then blot the roots dry with a paper towel.
4. Using the analytical balance, weigh the plant to the nearest .0001 gram.
Record this datum to your data sheet.
5. Return the plant to its labeled pot. Do not discard the plants; samples from
some roots will be used for the final part of this exercise.
D. STAINING AND VIEWING MYCORRHIZAE
Materials
forceps
gloves, safety goggles, aprons
5% (vol/vol) ink-vinegar solution made from:
Schaeffer black ink
white household vinegar, (5% acetic acid)
10% (wt/vol) KOH (potassium-hydroxide)
hotplates
beakers
tongs for lifting beakers
glass microscope slides and cover slipscompound microscopes
Procedure
The method of staining roots presented here is after Vierheilig et al. (1998).
Be sure to wear gloves, eyewear, and an apron during this procedure.
Obtain root samples from 1 plant per experimental treatment and stain them for viewing under the microscope. (If your group tested 2 replications of 2 species each of 2 different treatments, then you will have 8 root samples.) Root samples should be about 2 cm long from the tips of the root.
The percentage of plants staining successfully may be about 50%, so replicates are important for the success of this technique. In addition, by staining a number of samples, the percentage of roots with fungi present is a convenient way to summarize whether the soil harbors fungal spores and hyphae and/or whether the plant species is dependent or not on mycorrhizae for optimal growth. Therefore, the instructor will ask students to pool their data at the end of class.
1. Clearing roots:
a. rinse roots in tap water to clean off any residual soil particles
b. boil roots in 10% KOH for 3-5 minutes (longer for bean; shorter
for cucumber)
c. rinse roots in tap water
2. Staining roots: boil roots for 3 minutes in 5% ink-vinegar solution
3. Rinsing roots: rinse the roots in tap water + a few drops of vinegar for
20 minutes
4. Viewing roots:
a. make a wet mount of each root and view the root under a compound
microscope
b. roots should appear brownish-red and fungal structures should appear black
c. for each root sample determine if mycorrhizae are present
E. DATA ANALYSIS
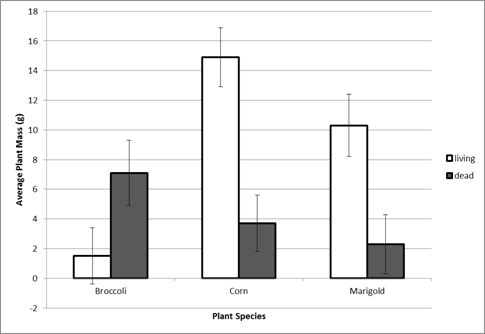
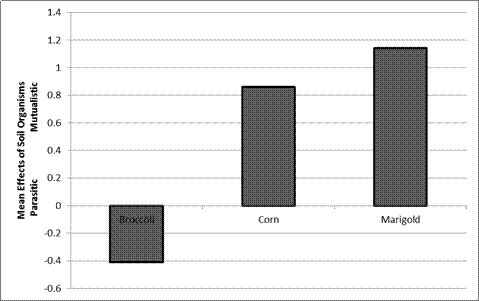
Make three bar graphs using your data: 1) soil treatment and plant species vs. plant height (cm), 2) soil treatment and plant species vs. total plant mass (g), and 3) plant species (mycorrhizal-dependent and non-mycorrhizal-dependent) vs. mean effects of soil organisms. See examples of what these graphs will look like in Figures 1 and 2 below.
To determine the mean effects of soil organisms on plant growth (i.e., is their effect mutualistic or antagonistic?), using the following equation (from Johnson et al. 2009):
Mean effects of soil organisms = (PLANT MASS experimental – PLANT MASS control)
If the plant mass with mycorrhizae (experimental) is greater than the plant mass when mycorrhizae are absent (control), the mean effects of soil organisms are positive, or mutualistic. On the other hand, if the plant mass with mycorrhizae (experimental) is less than the plant mass when mycorrhizae are absent (control), the mean effects of soil organisms are negative, or parasitic/competitive.
Share your results from the root staining and viewing procedure with the class. From the pooled data calculate the percentage of of roots with mycorrhyzae for each species under each treatment condition.
Figure 1. Soil treatment (living or dead) and plant species vs. plant weight (g).
Data redrafted from Johnson et al. 2009, Figure 4B. Corn and Marigold are mycorrhizal-
dependent species and broccoli is not. Note: the graph of soil treatment vs.
plant height (cm) will have a similar layout.
Figure 2. Soil treatment vs. mean effects of soil organisms. Data redrafted from
Johnson et al. 2009, Figure 4C. Corn and Marigold are mycorrhizal-dependentspecies and broccoli is not.
Questions
What effect, if any, did the addition of commercial inoculum (Arbico Organics Root Maximizer Mycorrhizal Fungi) have on plant growth? Would you recommend the use of inoculum to a forester or farmer?
Is there a difference in the response of mycorrhizal-dependent vs. non-mycorrhizal-dependent plant species to the presence of mycorrhizae in the soil? Explain, based on your data.
Are all soil fungi mutualistic? Name some other ecological roles that fungi perform in soils, providing actual examples to support your answer.
What other organisms might be present in soils? (Think broadly, in a taxonomic sense) Would you predict their effects on plant growth to be mutualistic, parasitic, competitive, or some combination? How could you tell if a particular effect of soil organisms on plant growth was the result of parasitism or competition?
REFERENCES
Johnson, N.C., V.B. Chaudhary, J.D. Hoeksema, J.C. Moore, A. Pringle, J.A. Umbanhowar, and G.W.T. Wilson. 2009. Mysterious Mycorrhizae? A field trip and classroom experiment to demystify the symbioses formed between planta and fungi. The American Biology Teacher 71: 424-429.
Vierheilig, H., A.P. Coughlan, U. Wyss, and Y. Piche. 1998. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied and Environmental Microbiology 64: 5004-5007.